Innovative range of integrated systems for pathology

The construction of Tissue Micro-Arrays (TMA) is a tedious task with many steps that are prone to errors.
MiniCore® 4 greatly simplifies operations by automating the construction of Tissue Micro-Arrays. The MiniCore®4 software displays all the real-time information. It performs the superposition and the registration automatic creation of virtual slides annotated on the image of the donor blocks using artificial intelligence, which allows very precise selection of coring areas.
MiniCore® 4 features a large loading capacity with 4 blocks receivers and 16 donor blocks per board. It also offers 4 games needles ranging from 0.6 mm to 2.0 mm in diameter.
Compact and ergonomic system
Advanced technology
Optimal traceability
Extended compatibility
Excilone®View software allows you to view and select areas of interest on scanned slides. Excilone®View is compatible with most slide scanners currently on the market. Try the free version of Excilone®View (Digital Pathology – Excilone).
Precision of tissue sampling
Large capacity
|
Description |
Reference |
|
0.6 mm “donor” needle |
MIN04-06D |
|
0.6 mm “receiver” needle |
MIN04-06R |
|
1.0 mm “donor” needle |
MIN04-10D |
|
1.0 mm “receiver” needle |
MIN04-10R |
|
1.5 mm “donor” needle |
MIN04-15D |
|
1.5 mm “receiver” needle |
MIN04-15R |
|
2.0 mm “donor” needle |
MIN04-20D |
|
2.0 mm “receiver” needle |
MIN04-20R |
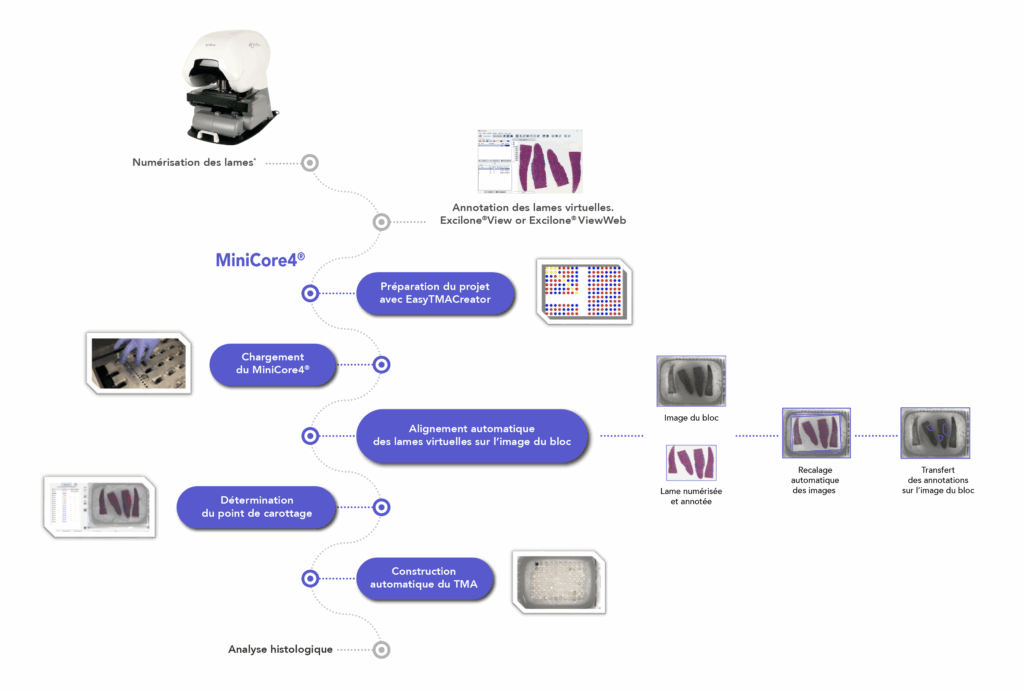
* Optional steps: only when the process is performed with scanned slides.
CE Marking : Built to CE/EMC requirements and certified to these standards.
|
Component |
Description |
|
Dimensions |
Width : 560 mm Depth : 720 mm Height : 650 mm |
|
Weight |
Approximately 45 kg |
|
Alimentation |
85/264 V~, 47-63 Hz, 150 VA |
|
Number of receiving blocks |
up to 4 |
|
Number of donor blocks |
up to 16 |
|
Available needle sizes |
0.6 mm / 1.0 mm / 1.5 mm / 2.0 mm |
|
Work surface on the block |
40 mm x 28 mm |
|
Thickness max. thickness |
10 mm |
|
Length of carrots |
Configurable on the software |
|
Coring area |
Manual determination on the block image. |
Indicative but non-contractual specifications.
|
Specifications |
MiniCore®3 |
MiniCore®4 |
|
Model |
Semi-automatic |
Automatic |
|
Size |
Width : 340 mm Depth : 400 mm Height : 300 mm |
Width : 560 mm Depth : 720 mm Height : 650 mm |
|
Integrated computer |
No |
Yes |
|
Maximum loading capacity |
8 blocks (Donor + recipient blocks) |
4 receiving blocks 16 donor blocks/run |
|
EasyTMACreator Software (Project design) |
Yes |
Yes |
|
Automatic blade/block overlay |
No |
Yes |
|
Traceability: – Image capture – Barcode reading – Anomaly monitoring – Excel tracking file |
Yes No No Yes |
Yes Yes Yes Yes |
|
Number and size of needles available |
3 needle sizes : – 0,6 mm – 1 mm – 2 mm |
4 needle sizes : – 0,6 mm – 1 mm – 1,5 mm – 2 mm |

MiniCore®3 includes an 8-position carousel as standard, allowing you to use up to 7 donor blocks simultaneously, saving you even more time and effort.
Core zone selection in donor blocks is performed by zone drawing or pointing, directly on screen on the donor block image superimposed on that of the H&E control blade.
The MiniCore®3 Control Station software interface displays all information in real time. In the event of difficulties on a donor block, the user can easily switch blocks and continue his tissue array, without any loss of quality.
With our TMA construction software, you can design any type of format from a list of Excel® specimen identifiers.
During construction, MiniCore® 3 automatically uses your tissue array configuration file. Position errors are virtually eliminated.
Whenever a donor block is required, its ID is displayed on the screen. Specimen errors are greatly reduced.
Finally, each step is recorded in a log file to guarantee perfect traceability.

Spot identification is undoubtedly the most time-consuming and difficult stage in preparing tissue array spots for analysis. Identification includes detecting spots on the slide scan, then associating each of these spots with their respective position in the initial plan of your Arrays tissue, to create the essential traceability link between spot and patient data.
Spot Browser® 4 includes a de-Arraying tool for data detection and association. Simply import your tissue array map file, and Spot Browser® 4 takes care of the rest. Interactions and corrections are always possible in cases where the Tissue Array is truly in poor condition on the blade (severe deformations).
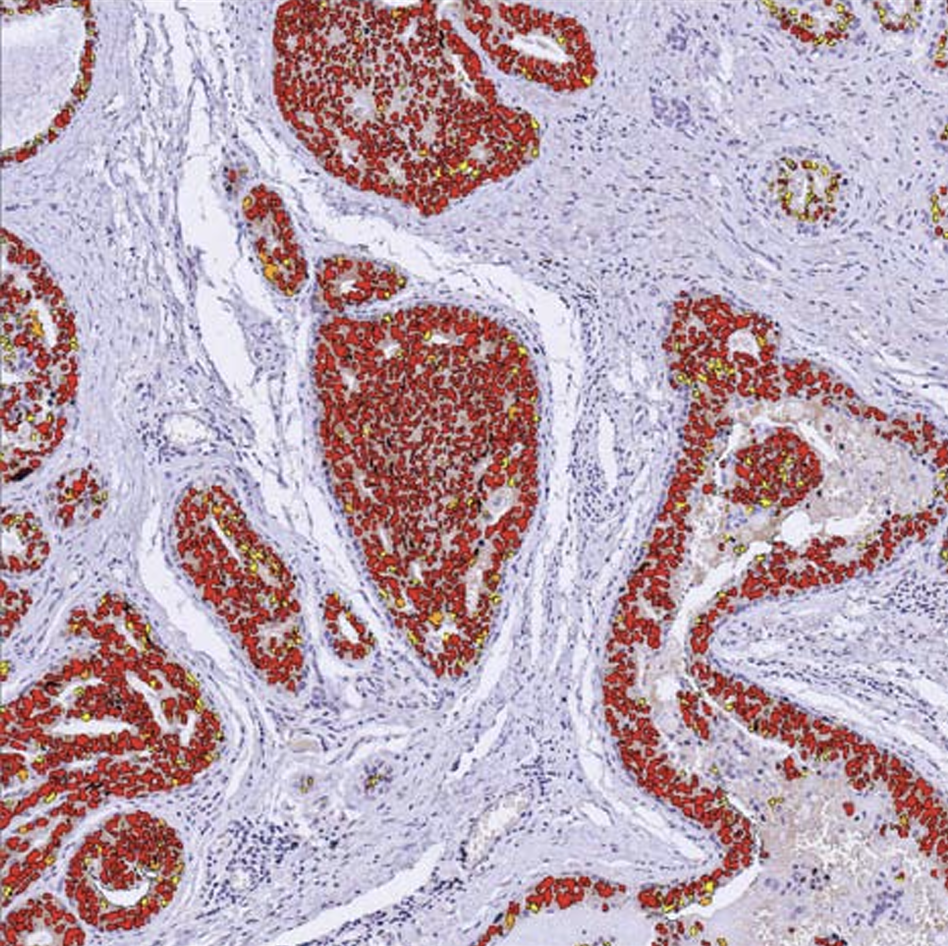
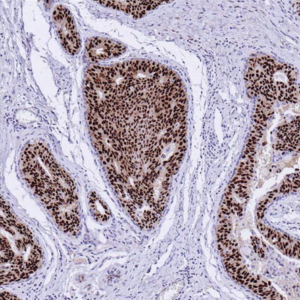
In many cases, spots are “scored” visually, because the pathologist’s expert eye cannot so easily be replaced by software, however powerful.
To meet this need, Spot Browser® 4 allows users to create their own data fields in various formats (free, numeric, drop-down list…) to enter their annotations. The user can quickly navigate from one spot to another and enter annotations.
Input and imported data can be exported in Excel format for statistical processing.
For analysis of large numbers of spots or for quantitative analysis, Spot Browser® 4 offers a comprehensive tool for automatic image analysis.
HSV color and morphometry are the characteristics used. Each of these features can be used alone or in combination, depending on the objects to be detected.
Users can easily create their own detection protocols, with one or more types of object to be detected, and with or without nesting, for example, to count labeled nuclei in a tumor zone. Cytoplasmic and membrane labelling can also be detected.
Detection and analysis protocols can be saved in internal libraries for future use.
The user can analyze spots one by one or in automatic batch mode, and analyses can combine human intervention (zone tracing, for example) with fully automatic modes.
Raw and interpreted results can be exported to Excel.
 SnapFrost® guarantees ultra-fast, reproducible freezing of specimens.
SnapFrost® guarantees ultra-fast, reproducible freezing of specimens.The reference technique using Iso pentane refrigerated down to -80°C, which unlike liquid nitrogen does not degas, enables rapid, reproducible temperature transmission to the sample.
Molecular content does not diffuse into the sample, cell membranes are preserved and tissue damage is avoided.
Ergonomic and flexible in use, SnapFrost® features two individually controllable freezing chambers to suit all freezing protocols and tissue types. The volume of the lower chamber containing Iso pentane can be modulated (from 5 to 680 ml) to suit all sample sizes.
Alternative solutions such as the Novec 7100 can also be used in the SnapFrost®, retaining the advantages of the appliance and guaranteeing high quality freezing.
Programmable, compact and autonomous, SnapFrost® can be moved to different freezing zones, guaranteeing flexibility, time savings, safety, quality and reproducibility in your freezing operations.
Traditional tissue controls have long been the standard in immunohistochemistry, but they have limitations: lack of standardization, batch-to-batch variability, and risk of misuse. PRS slides are revolutionizing immunohistochemistry by replacing these tissue controls.
Standardizing the IHC staining process using the PRS tool offers several advantages in antibody validation:
Quantitative assessment : Predetermined quantification of surrogate antigens allows for quantitative assessment of staining intensity, thus facilitating objective evaluation of the performance of the antibodies used.
Quality control : The PRS tool serves as a quality control measure, allowing users to verify the reliability and consistency of antibody staining between different lots and experiments.
Error detection : Deviations between the observed staining pattern and the expected result based on the PRS tool indicate potential errors in antibody selection or application, prompting further investigation and corrective action.
Workflow efficiency : Standardizing the staining process streamlines workflow procedures, reduces variability, and optimizes laboratory efficiency in IHC testing; no more control tissue pattern to deposit on the slide in addition to the test tissue.
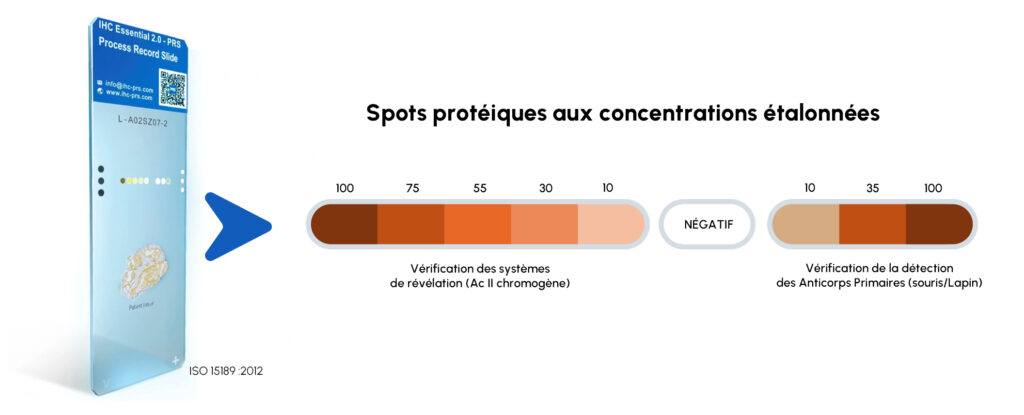
PRS eliminates false negative/positive staining results with 100% slide control; each IHC slide is evaluated individually.
PRS allows you to avoid expensive commercial control blocks.
PRS ensures accurate and consistent results while meeting ISO 15189 standards. Primary and secondary protein targets with certified antigen expression levels are recognized as an ISO 13485 medical device. PRS helps achieve accreditation and regulatory compliance with ISO 15189, CLIA, and ACP standards.